Thus, the presence of antioxidants while in the sample interrupts the oxidation of fluorescein, extending the length with the fluorescein’s glow. By checking the response over time, researchers can determine the antioxidant capability on the sample based upon the rate that fluorescein loses its glow.
A response by which the analyte and titrant kind an insoluble precipitate can also function The premise to get a titration. We call such a titration a precipitation titration.
Questions according to precipitation titration are sometimes asked in assessments and exams. So, by Mastering this concept, you should be able to response these queries and safe an excellent score.
Titration is a way Employed in analytical chemistry to find out the focus of an unidentified Answer by using an answer of regarded concentration. Answer of recognized focus is named titrant when the answer of unfamiliar concentration is known as analyte in titration system. Precipitation titration is often a form of titration which requires the formation of precipitate all through titration for the endpoint.
The quantity improve from the common Alternative at which the end level takes place is pointed out. This volume indicates the quantity of titrate that's been utilized to neutralize the analyte. These values are then Employed in further calculations.
The food items market depends on redox titration for top quality control and deciding nutrient content material. Exploration in different industries usually will involve redox titration for procedure optimization. In instructional settings, redox titration can help students fully grasp chemical reactions.
This technique was supplied by American chemist Kazimierz Fajan. That’s why it is called fajan’s strategy. This process is generally known as the indicator adsorption approach for the reason that in this technique chloride ions present in excess are adsorbed on silver chloride surface.
On account of their steadiness towards air oxidation, most redox titrations use an oxidizing agent as being a titrant. Titrations with decreasing brokers are also possible. Precipitation titrations generally contain Ag+ as either the analyte or titrant.
A second kind of indicator takes advantage of a more info species that varieties a coloured advanced with the titrant or perhaps the titrand. Within the Volhard approach for Ag+ employing KSCN as the titrant, as an example, a little quantity of Fe3+ is included to your titrand’s Remedy.
Acid–foundation titrations rely on the neutralization among an acid as well as a base when blended in Option. In combination with the sample, an suitable pH indicator is additional towards the titration chamber, representing the pH variety of the equivalence position. The acid–base indicator signifies the endpoint from the titration by altering colour. The endpoint as well as the equivalence stage are not exactly the same because the equivalence issue is determined via the stoichiometry with the reaction when the endpoint is just the color alter from the indicator.
Redox titration website includes an oxidation-reduction process concerning two chemical species: an oxidizing agent in addition to a cutting down agent. During such a titration, electrons are transferred involving reactant ions in aqueous methods.
Other types of titrations can measure the concentration of major metals, pesticides, and natural compounds in environmental samples; scientists use this details to observe environmental regulation compliance in the area and recognize resources of contamination.
It proceeds until the last amount of analyte is eaten. It truly is utilized to ascertain chloride by utilizing silver ions.
To research the consequences of a few titration methods on correct coronary heart operate and prognosis in people with ARDS.
 Neve Campbell Then & Now!
Neve Campbell Then & Now! Mackenzie Rosman Then & Now!
Mackenzie Rosman Then & Now! Nancy McKeon Then & Now!
Nancy McKeon Then & Now!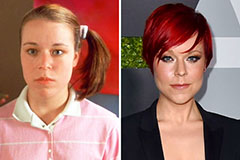 Tina Majorino Then & Now!
Tina Majorino Then & Now! Andrew McCarthy Then & Now!
Andrew McCarthy Then & Now!